Thursday, September 16, 2004
Surprise, Surprise
Unintended Consequences of FDA Antidepressant Meeting
By Val Brickates Kennedy, CBS.MarketWatch.com
Last Update: 6:16 PM ET Sept. 14, 2004
BOSTON (CBS.MW) -- Shares in several of the largest producers of antidepressant drugs rose Tuesday as U.S. Food and Drug Administration hearings revealed no startling new data linking the use of antidepressants by children with suicidal behavior.
Shares of pharmaceutical giants Bristol-Myers Squibb (BMY: news, chart, profile), Eli Lilly (LLY: news, chart, profile), Forest Laboratories (FRX: news, chart, profile), GlaxoSmithKline (GSK: news, chart, profile), Pfizer (PFE: news, chart, profile) and Wyeth (WYE: news, chart, profile) were all on the move, as an FDA advisory panel heard testimony on the safety of prescribing antidepressants to children and adolescents. See full story.
Late Tuesday, a special panel convened by the FDA recommended that all antidepressants have expanded labeling warning physicians and parents that use of the drugs has been linked to suicidal behavior in some youngsters. The warning would also extend to Eli Lilly's Prozac, which is currently the only antidepressant approved in the United States for treating depression in children.
The panel also said that while certain antidepressants could trigger
suicidal tendencies in a small number of patients, the drugs can also
be effective in treating pediatric depression.[...]
According to analysts, the FDA's hearings did not reveal anything
that industry watchers didn't already know.
"I'm not sure what people were expecting," said Thomas Weisel analyst
Donald Ellis. "They shouldn't have been that worried about the data
that was already out there."
From the "full story" that the first story refers to:
FDA could seek more warnings on nine antidepressants
By Laura Gilcrest, CBS MarketWatch
Last Update: 6:56 PM ET Sept. 14, 2004
WASHINGTON (CBS.MW) -- A revamp of labels on widely prescribed antidepressants gained momentum when a Food and Drug Administration advisory panel Tuesday said that regulators should add a warning to the drugs, advising physicians of an increased risk of suicide behaviors in pediatric patients.
[...] However, the panel shied away from a more drastic option up for review: advising that antidepressants not be prescribed to children at all. Britain recently banned use of the drugs in pediatric patients, panelists noted.
The panel also said the drugs' sponsors should do more pediatric studies of antidepressants. The FDA is not bound to follow its panel's advice but does so in most cases.
For many children, however, the benefits of taking the drugs may outweigh the risks, the agency said earlier in the day, asking the panel of experts for input on whether the drugs' labels ought to be changed to reflect that. [...]
The data showed that, for every 100 children or adolescents taking antidepressants, there is a risk that about two or three patients will show increased suicidal behaviors caused by the drugs and not the child's underlying depression, Laughren told the advisory panel.
Nevertheless, the data suggest that roughly 25 percent of these 100 patients will get a benefit from taking antidepressants, he added. Thus, the use of these drugs by children requires doctors to carefully balance an antidepressant's risks and benefits. [...]
Separately, the FDA's Robert Temple told the panel that the agency may start requiring companies to add pediatric studies to their drugs' labeling, showing the drug doesn't work in children.
Yesterday, I stated that the FDA had clarified the situation. I guess I spoke too soon: now we hear that some panel members say that the medications can help, but one says that they don't work. How is it that different experts looking at the same data could derive seemingly opposite conclusions?
To understand this, it is necessary to understand how the results of antidepressant drug studies are evaluated. During the study, the patients are assessed using numerical rating scales. The score is supposed to correlate with the severity of the depression. An example is the HAM-D, or Hamilton Rating Scale for Depression. The drug company Glaxo kindly posted a copy on the 'net. The HAM-D is used commonly in the USA. European studies tend to use the MADRAS (Montgomery-Asberg Depression Rating Scale). The patients then are divided into two groups. One groups gets active drug; the other groups get placebos.
The scales are administered periodically throughout the study. At the end of the study, a patient is considered to be a "responder" if the final score is less than 50% of the initial score. Invariably, some patients will get better; some will get worse. This will be true no matter what drug is given, or even if the patient is given placebo.
In order to determine if the drug "works," the initial scores are added up, and the arithmetic mean is calculated. Then the mean final score is calculated. This is done separately for the patients given active drug and those given placebo. The a statistical analysis is done. If there is a greater drop in the mean scores for the drug-treated patients than for the placebo-treated patients, AND if the difference is statistically significant, then the drug could be said to be effective.
Note that you might get a statistically significant difference even if the HAM-D scores only go down by an average of one point. For that reason, the mere presence of a statistically significant difference is not enough for doctors to want to prescribe a drug. Therefore, additional analysis of the data is performed. The percentage of patients who are responders is calculated for each group. In order for an antidepressant to be considered truly effective, there has to be a significant difference in the rates of drug response. This is a way of assuring that the effect size is clinically meaningful.
Those readers who are familiar with statistics will see that a great deal of information is lost, if all you look at is the presence or absence of a statistically significant difference in the rate of response between the two groups.
One problem with the statistical analysis of antidepressant study data is that there is always a large placebo response rate. That is, usually you get a response in 30 to 40% of the patients who take placebo.
Now, to illustrate a point, let's assume that a study is done, and that 35% of the patients in the placebo group are responders, and 60% in the drug group are responders. Let us say that an analysis of the data shows that there is a 10% chance that that the difference could be due to random variation. That would not meet the traditional criterion for statistical significance, yet it is clear that, on average, the patients who got the drug did better than those who did not.
One way to interpret the data would be to say that the drug helped about 25% of the patients, based upon the idea that 35% would have gotten better anyway, but the rest of the 60% who got better, got better because of the drug.
The panel members who stated that the drugs can work were looking at it that way.
Another way to interpret the data would be to say that the usual standard for accepting a study is to accept it as a success only if it is statistically significant at the 95% confidence level. Since the studies did not show that, one could conclude that the drugs don't work. Same data; different conclusion.
Of course, if the drug never caused adverse effects, most people probable would accept a 25% chance of response, if that is the best that medical science had to offer. But because the studies show that there is a 2 or 3% chance of the drug causing suicide-related behavior, the situation becomes much more complex. Add to that the fact that untreated depressed patients also have a risk of suicide and suicide-related behaviors, and the risk-benefit decision becomes murky.
Traditionally, the US government has tried to leave these kinds of risk-benefit decisions to the doctors and their patients (or parents, in the case of children.) The government lets people over the age of 18 smoke cigarettes, so long as there is a warning label. It lets people over the age of 16 drive a car, so long as they have passed a test that assures they know the risks they are taking. It does not let people inject cocaine into their veins, and a warning label does not make a difference. Thus, the role of the FDA is to decide which risks should be left to the individuals involved, and which risks it simply will not allow. That is what the recent FDA committee meeting set out to decide.
In this case, they took the middle ground: it is OK to let people take the risk, so long as the warnings are made clear. They also added a lukewarm acknowledgment that the drugs might be effective, at least for some people, to an extent that some reasonable people might decide that the chance of a benefit is worth the risk. The stock market responded to this lukewarm endorsement, and the drug company stocks went up.
Of course, the US government traditionally has allowed people to take risks in the stock market.
(Note: The Rest of the Story/Corpus Callosum has moved. Visit the new site here.)
E-mail a link that points to this post:
Monday, September 13, 2004
Sorting Antidepressant Evidence is Tough
Google lists 133 news articles already. The best title I saw was this one:
Dallas Morning News (subscription), TX -
By KAREN PATTERSON / The Dallas Morning News. Drug regulators are ready to make hard decisions about the safety of antidepressant medicines. ...
Other article titles are : FDA Panel Debates Suicide Risk for Kids On Antidepressants; Mom Credits Prozac with Saving Child's Life; Reviewer Says Depression Drugs, Suicide Linked. Notice that the titles span a spectrum from "Yes, there is a risk;" to "The risks are debatable;" to "We can't tell if there is a risk;" up to "These drugs saved my child." The consumer, meanwhile, has to sort this out.
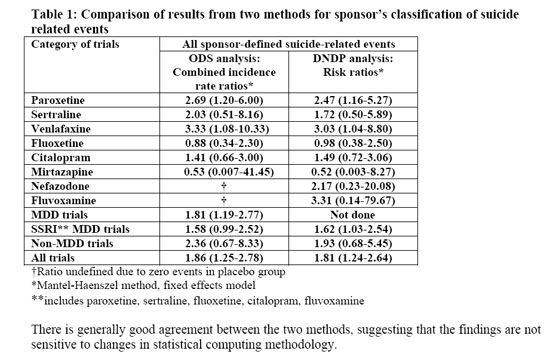
I did not read all 133 articles, but a brief survey indicates that there is no new information yet, compared to the material already available. The FDA posted the briefing materials for the meeting ahead of time. There are two pages: 1 2. Most of the information is on links from the first page. Much of the information is esoteric; for example, here is a table from a summary PDF document:
The two data columns refer to the original statistical analysis of the data done by the Office of Drug Safety. When there was a lot of political turmoil, the FDA asked a group at Columbia University to reclassify the data regarding "suicide-related" adverse events. This was done because it is not always a simple matter to classify such events. For example, if someone takes five aspirin tablets, is that a suicide attempt? The reason for analysis of suicide related events, rather than actual suicide, is that there were no actual suicides in any of the studies. The Division of Neuropharmacological Drug Products then analyzed the data again. The results are roughly comparable.
OK, the data are comparable. But what do they mean? A risk ratio is a number that indicates relative risk, not absolute risk. The number "2.69," for example, in the ODS analysis of the date for paroxetine (Paxil) shows that for every one suicide-related event per unit of time in the placebo group, there were 2.69 such events in the group that took the active drug. The numbers in parentheses denote the confidence interval. For examples of how these numbers can be misinterpreted, follow the link to the Wikipedia article on confidence intervals. Unfortunately, the text of the article does not say what level of confidence is indicated by the intervals, but they probably mean 90% confidence. For the paroxetine data in the ODS analysis, the numbers indicate that we can be 90% sure that the actual risk ratio is between 1.20 and 6.00. In other words, there is only a 5% chance that the relative risk is less than 1.2. Therefore, it is reasonable to conclude that this represents a real increase in risk.
Unfortunately, the real question here cannot be answered with the data. That is: is there a correlation between the suicide-related behaviors observed, and any risk of actual suicide? Until we know more, I think we have to assume that there is a correlation, but it would be wise to remember that this has not been proven. At first, it may seem like a no-brainer, in that it seems so plausible to assume that there would be a direct connection. Remember, though, that we are talking about life-or-death situations here, so we really would like to know for sure.
Since right now the evidence for the effectiveness of antidepressant medication, for the treatment of major depression in children, is weak, probably most consumers would want to avoid such use in the treatment of mild or moderate cases. In more severe cases, one would want to exercise caution, but, depending on a variety of factors, decide to go ahead with it. This probably is what the FDA will conclude, once they finally get around to making a clear statement of the subject.
(Note: The Rest of the Story/Corpus Callosum has moved. Visit the new site here.)
E-mail a link that points to this post:
Sunday, September 12, 2004
Neuropeptide S and Me
 The story begins in
1980. That summer, I was in Wyoming, collecting Eocene
vertebrate
fossils for Dr. Philip
Gingrich's
team of paleontologists from the University of Michigan. I was
just along for the ride, really, but I did find a lot of fossils.
My new wife was a graduate student in physical anthropology at the
time, and had to do some fieldwork. Living in a tent in the
Wyoming badlands is not the usual idea of a great honeymoon --
unless you're a scientist.
The story begins in
1980. That summer, I was in Wyoming, collecting Eocene
vertebrate
fossils for Dr. Philip
Gingrich's
team of paleontologists from the University of Michigan. I was
just along for the ride, really, but I did find a lot of fossils.
My new wife was a graduate student in physical anthropology at the
time, and had to do some fieldwork. Living in a tent in the
Wyoming badlands is not the usual idea of a great honeymoon --
unless you're a scientist. I had a great time.
 The
year before, I had decided to go to medical school. My other
career choice had been bioengineering, but I chose medicine because it
is the more portable of the two professions. One of us had to do
something practical, as anyone married to a grad student knows. I
knew I was about to spend eight years with my nose in a book. A
summer in Wyoming was the perfect experience to prepare for the grind.
The
year before, I had decided to go to medical school. My other
career choice had been bioengineering, but I chose medicine because it
is the more portable of the two professions. One of us had to do
something practical, as anyone married to a grad student knows. I
knew I was about to spend eight years with my nose in a book. A
summer in Wyoming was the perfect experience to prepare for the grind.While I was in Wyoming, a country singer named Merle Haggard had a hit song: Big City.

Entirely too much work and never enough play.
And I'm tired of these dirty old sidewalks.
Think I'll walk off my steady job today.
Turn me loose, set me free, somewhere in the middle of Montana.
And gimme all I got comin' to me,
And keep your retirement and your so called social security.
Big City turn me loose and set me free.
Been working everyday since I was twenty.
Haven't got a thing to show for anything I've done.
There's folks who never work and they've got plenty.
Think it's time some guys like me had some fun.
Turn me loose, set me free, somewhere in the middle of Montana.
And gimme all I got comin' to me,
And keep your retirement and your so called social security.
Big City turn me loose and set me free.
That sort of sums up how I felt.
Neuroscience was in an early stage of development. For example, the dopamine hypothesis of schizophrenia was just being established, and there were two competing hypotheses to explain major depression. Some thought is was primarily a deficiency of norepinepherine in the brain; others thought that serotonin was the culprit. We had just learned about neurotransmitters and receptors, and it was becoming possible to do quantitative assays of neuroreceptors. Because of these and other related discoveries, it seemed that we were on the verge of resolving the scourge of mental illness. Since that time, we indeed have made some major advances, but the field of neuroscience has turned out to be much more complex than anyone imaging at the time.
Being a bit of an idealist, once I got to medical school, I thought I would end up as a family practitioner or a pediatrician. However, as I got more clinical experience in the third and fourth years, I developed the impression that I did not want to spend every day seeing one ear infection after another. That is an unfair characterization of primary care, but it's what I was thinking at the time. I had learned about the dopamine hypothesis and receptors and all that, and I knew that psychiatry was about to undergo a major transformation. Not wanting to be stuck in a boring profession, I decided to go into psychiatry.
You see, at the time, it seemed to me that being a pediatrician would be like having a job in a big city. Just one little problem after another, never really getting anywhere. Psychiatry, on the other hand, offered the lure of being "somewhere in the middle of Montana:" wide open horizons, a new discovery everywhere you look.
Of course, I was right about a couple of things. Psychiatry -- which is really the applied branch of neuroscience -- is undergoing a major transformation. It is not boring. Every time I think to myself, "now I've seen everything,' someone with something new comes into the clinic. There is no end to human variation. Having the time to really get to know patients, seeing all the little details that make up a complex life story; these factors lead to endless fascination.
In contrast, I was wrong about a couple of things. We were not on the verge of eliminating the scourge of mental illness. We were not close to finding some kind of ultimate truth about the causes and cures. Instead, what has happened, is that every question that gets answered, merely leads to more questions.
In the time since 1980, we have discovered many "new" neurotransmitters. We have learned that each transmitter acts on a variety of different receptors, and that these receptors are made and regulated in complex, mysterious ways. For example, the GABA type A receptor is made of five protein subunits. Each person has multiple slightly different genes that code for the subunits. If you calculate all the possible permutations, it turns out that there are over 1000,000 different ways to make that one receptor. Complexity always has purpose in biology, so there must be a reason for that complexity to exist. It's just that we have only the vaguest notion why.
Each discovery leads to speculation and hope about possible clinical applications. Neuropeptide S (NPS) is a good example. The August 19, 2004 issue of the journal Neuron has an article about NPS. I can't legally reproduce the whole thing here, but here is the abstract:
Neuron, Vol 43, 487-497, 19 August 2004
Neuropeptide S: A Neuropeptide Promoting Arousal and Anxiolytic-like Effects
Yan-Ling Xu 16 , Rainer K. Reinscheid 16 , Salvador Huitron-Resendiz 4, Stewart D. Clark 1, Zhiwei Wang 1, Steven H. Lin 1, Fernando A. Brucher 2, Joanne Zeng 1, Nga K. Ly 1, Steven J. Henriksen 4, Luis de Lecea 5, and Olivier Civelli 1,3
1Department of Pharmacology, University of California Irvine, Irvine, CA 92697 USA
2Department of Psychiatry and Human Behavior, University of California Irvine, Irvine, CA 92697 USA
3Department of Developmental and Cell Biology, University of California Irvine, Irvine, CA 92697 USA
4Department of Neuropharmacology, The Scripps Research Institute, La Jolla, CA 92037 USA
5Department of Molecular Biology, The Scripps Research Institute, La Jolla, CA 92037 USA
Correspondence:
Rainer K. Reinscheid
(949) 824-9228 (phone)
(949) 824-4855 (fax)
rreinsch@uci.edu
Arousal and anxiety are behavioral responses that involve complex neurocircuitries and multiple neurochemical components. Here, we report that a neuropeptide, neuropeptide S (NPS), potently modulates wakefulness and could also regulate anxiety. NPS acts by activating its cognate receptor (NPSR) and inducing mobilization of intracellular Ca2+. The NPSR mRNA is widely distributed in the brain, including the amygdala and the midline thalamic nuclei. Central administration of NPS increases locomotor activity in mice and decreases paradoxical (REM) sleep and slow wave sleep in rats. NPS was further shown to produce anxiolytic-like effects in mice exposed to four different stressful paradigms. Interestingly, NPS is expressed in a previously undefined cluster of cells located between the locus coeruleus (LC) and Barrington's nucleus. These results indicate that NPS could be a new modulator of arousal and anxiety. They also show that the LC region encompasses distinct nuclei expressing different arousal-promoting neurotransmitters.
For those without a subscription to Neuron, there are summaries posted elsewhere (1 2 3 4). The company that makes NPS (for research, not clinical use) has information about the experiments here.
Already, this has made it to the Blogosphere; Blogpulse lists four references (1 2 3 4). The sentiment expressed in these is illustrated by the following quotes:
- Cool beans, I want my new study drugs!!!
- Personally, I think that Olivier's newest paper (Xu et al., 2004, Neuron 43:487-497) on the discovery of a putative role for Neuropeptide S in arousal is a highly significant and exciting development.
- All I want to know is, when can I get some?
- Brain Protein Turns on Calm Alertness:
Finding could lead to treatments for sleep disorders and anxiety.
In 1980, I might have said something like "cool beans!" Now, I have a more reserved, stoical, skeptical response...not! It is really cool to think that we might be able to use this discovery to develop a drug that would simultaneously lower anxiety AND increase alertness. For example,. it might allow our military pilots to fly all night and not drop bombs on Canadian troops.
One interesting thing about finding is that it illustrates the falsity of a common misconception about psychiatric medication. It is believed commonly that such drugs are either "uppers" or "downers." Although that was true until the mid-1950's, in 2004 it is a gross oversimplification. So much so, that it is more misleading than useful.
The therapeutic applications for drugs that affect the NPS system could include: Attention Deficit Hyperactivity Disorder, sleep disorders, and anxiety disorders. Currently, the most effective drugs for these conditions all have some addictive potential. It is possible that an NPS receptor agonist could treat these conditions without the potential for drug dependence.
There are many hurdles to get over before anything clinically useful comes of this. First, since NPS is a peptide; therefore, it cannot be given orally. Even a peripheral injection probably would not get into the brain. The rats in the Xu study got NPS via intracerebroventricular (ICV) injection: it was injected into the cerebrospinal fluid; this is not acceptable for routine use by patients. What is needed is a chemical that can be given in pill form, get absorbed, make it through the hepatic portal system without all of it getting enzymatically degraded, pass the blood brain barrier, and get to the correct receptors -- all without killing the patient. Plus, there is no guarantee that the drug would do the same thing in humans that it does it rats.

Such concerns will not stop pharmaceutical companies from trying. Especially since this research could be used to produce a drug that could enhance the productivity of its own employees. Talk about return on investment!
Seriously, a medication that enhances alertness and decreases anxiety would raise some ethical issues. Some bioethicists, such as those on the President's Council on Bioethics, would suggest that the concept of "human dignity" precludes the use of drugs that enhance performance without actually treating a disease. Even if the drug turns out to have a useful purpose in the treatment of disease -- such as Narcolepsy -- the would argue that it should be used only to treat that disease. That is only one view espoused by conservatives. The opposite view is expressed by the National Center for Policy Analysis and the American Enterprise Institute:
Patients with genuine psychiatric problems that affect behavior should certainly have access to medications and third party coverage for their care, says Sally Satel.
Additionally, responsible adults who could benefit from these drugs should have access at their own expense.
They argue that such drugs should be available to anyone who wants them -- and who chooses to pay for them. Their only concern is that insurance companies should not have to pay, if it is not a "genuine" disease that is being treated. They do not address the question: Who gets to decide what constitutes a real disease? My answer, after 14 years of medical practice, is that the people who get to decide should be the doctors and their patients.
Even within the medical profession, this is a topic of some debate. For example, see the Medical Crossfire article about Cosmetic Psychopharmacology.
See what I mean? There is a new discovery everywhere you look, but every answer just brings more questions. And they are not always the questions that you would expect.
(Note: The Rest of the Story/Corpus Callosum has moved. Visit the new site here.)
E-mail a link that points to this post: